Peptide Studio
Services for predicting the effects and activities of dipeptides and tripeptides to reduce the time and effort of researchers to the utmost and to offer better outcomes.
Our service uses the Protein & Hit Method (PH Method), an algorithm that PH Japan has developed to predict if candidate sequences are effective, as well as to predict active peptide types.
Our analytical service examines two elements, the interlocking capability of amino acids and the balance as a dipeptide.
Table A on this page shows the interlocking capability of amino acids.
Verifications demonstrate the proportional relationship between the interlocking capability and activities of amino acids.
This page explains this relationship.
There is a trend where dipeptides in blue have relatively higher activities whereas those in red have relatively lower activities.
Table A
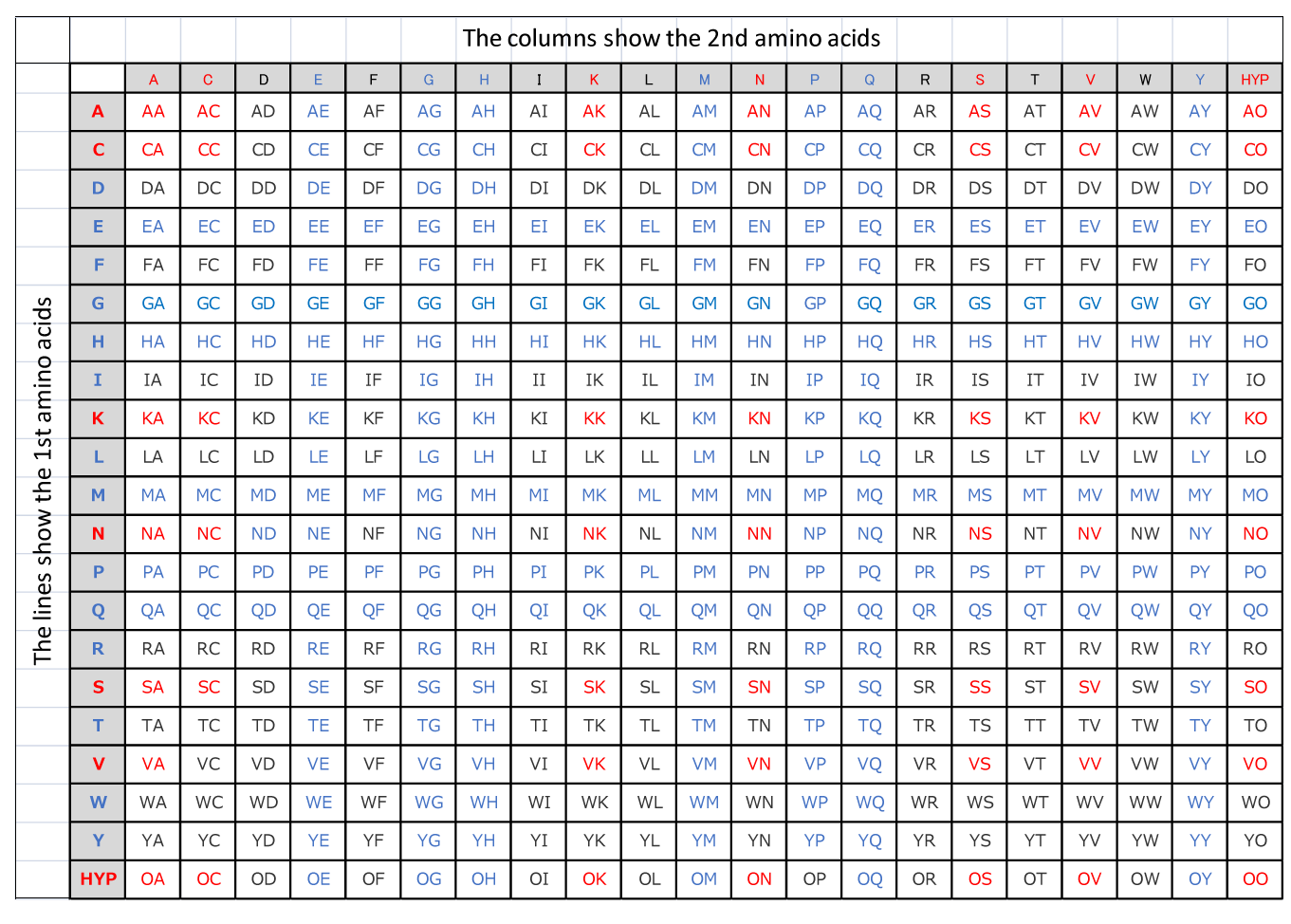
※Amino acid sequences with Q at the N terminal are structurally unstable.
All rights are reserved by PH Japan.
The interlocking capability of amino acids between dipeptides and tripeptides
The characteristics of amino acids are processed with the PH method and presented in Table B. First, this section explains the dipeptide-tripeptide interlocking capability using this table.
Table B
| A | C | D | E | F | G | H | I | K | L | M | N | P | Q | R | S | T | V | W | Y | HYP | |
| 1 | |||||||||||||||||||||
| 2 | |||||||||||||||||||||
| 3 | |||||||||||||||||||||
| 4 | |||||||||||||||||||||
| 5 | |||||||||||||||||||||
| 6 | |||||||||||||||||||||
| 7 | |||||||||||||||||||||
| 8 | |||||||||||||||||||||
| 9 | |||||||||||||||||||||
| 10 | |||||||||||||||||||||
| 11 | |||||||||||||||||||||
| 12 |
All rights are reserved by PH Japan.
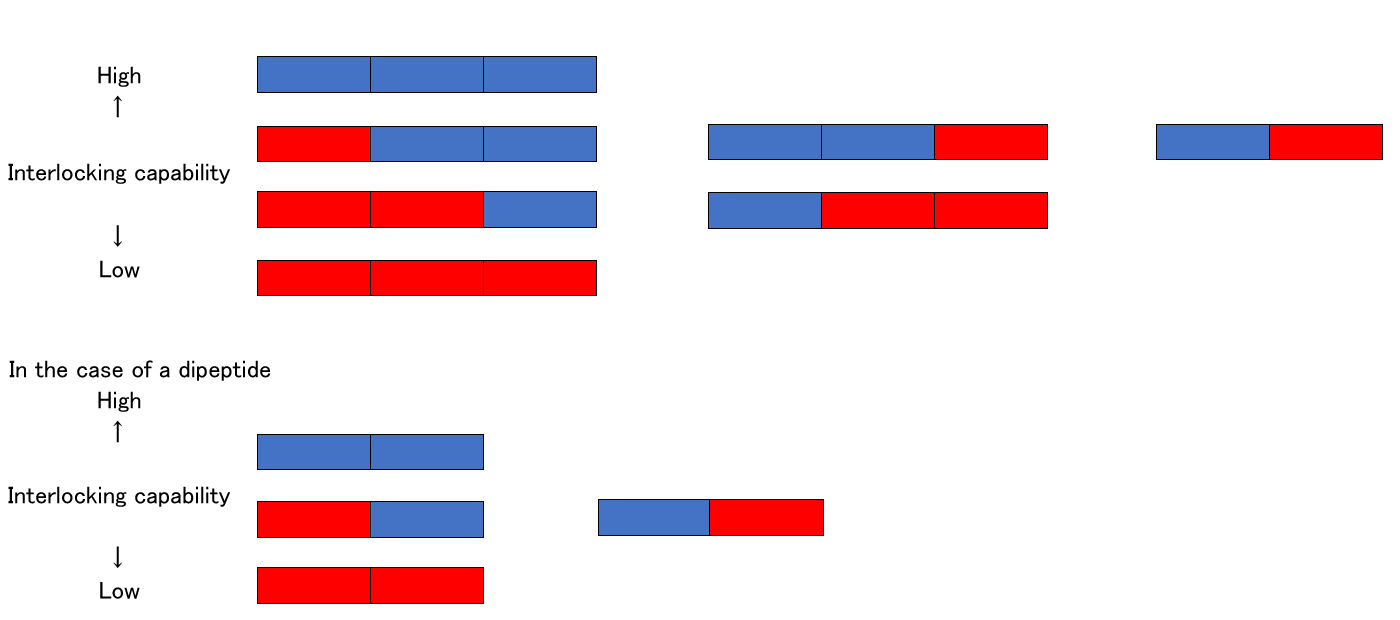
Amino acid A has four colored square boxes when counting vertically.
Amino acid G has only one box. Consider these square boxes as arms of a given amino acid.
On the basis of the above description, the dipeptide AG has four arms and one arm, respectively. All amino acids link with the adjacent amino acid with only a single arm. This means four combinations are possible for dipeptide AG
In the case of dipeptide AC, A has four colored square boxes, which makes 12 types of dipeptide AC combinations possible.
When compared with four types of AG these 12 types of AC combinations involve more complicated processes of linking amino acids with a single arm, which can reduce the interlocking capability between peptides.
The interlocking capability of dipeptides and tripeptides can be illustrated below:
In the case of a tripeptide
All rights are reserved by PH Japan.
Verifications of the proportional relationship between the interlocking capability of peptides and activities
PH Japan conducted comprehensive verifications using long-sequenced, physiologically active peptides.
Furthermore, the verifications used a variety of physiologically active peptides including Urocortin and other peptides to minimize bias in our verification efforts.
The results demonstrated that there were hardly any sequences with three sequential residues of lower interlocking amino acids in red, and one residue or at most two sites of sequences with two sequential residues of red amino acids in the case of Urocortin, for example.
Urocortin
Asp-Asn-Pro-Ser-Leu-Ser-Ile-Asp-Leu-Thr-Phe-His-Leu-Leu-Arg-Thr-Leu-Leu-Glu-Leu-Ala-Arg-Thr-Gln-Ser-Gln-Arg-Glu-Arg-Ala-Glu-Gln-Asn-Arg-Ile-Ile-Phe-Asp-Ser-Val-NH2
The ratio of amino acids in blue to those in red as shown in Table B is 14:7, while the ratio of Urocortin is 28:8. The proportion of active amino acids in Urocortin in blue is clearly larger as shown in this table, and this is almost true for other physiologically active peptides, thus indicating that there is a proportional relationship between interlocking capability and activities.
Working process of service for predicting effects and activities of dipeptides
The interlocking capability between peptides is already explained above.
This analysis examines two types of balance in dipeptides.
Dipeptides remain balanced by complementing deficits of amino acids.
More specifically, in the case of imidazole dipeptide which consists of β-alanine and histidine, AH is shown in blue in Table A, which means the dipeptide has relatively higher effects and activities, and anserine and carnosine are well-balanced.
These results allow the prediction that the dipeptide may have relatively higher effects and activities.
However, when taking the dipeptide AM as an example, although the dipeptide appears to have relatively higher effects and activities according to Table A, it lacks balance and thus is estimated to have lower effects and activities. This is the working process of our service.
Service for predicting effects and activities of tripeptides
Tripeptides have sequences with one extra residue compared with dipeptides and the analysis can include one additional element. The additional element will considerably enhance the precision and allow determinations for the presence of useful effects and activities by sequence.
For approximately 5 residues or more, the additional analytical element of symmetry can permit amino acid substitutions, etc., along with the targeted activity axis. For more details,please see the page for the physiologically active peptide service.
Please click here for inquiries.
 +81-82-223-6347
+81-82-223-6347